IGCSE Chemistry (C3 The Periodic Table and Equations) Note on Groups and Trends, created by ShreyaDas on 01/04/2014.
Pinned to
283
0
0
No tags specified
|
|
Created by ShreyaDas
about 11 years ago
|
|
Rate this resource by clicking on the stars below:




 (0)
(0)
Ratings (0)
| 0 | ||
| 0 | ||
| 0 | ||
| 0 | ||
| 0 |
0 comments
There are no comments, be the first and leave one below:
Close
700883
note
2016-09-20T00:30:43Z
1/1
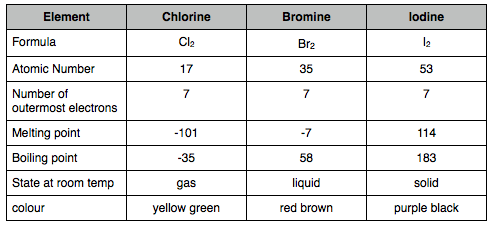
Melting and boiling points increase as you go down the group
7 electrons in the outer shell (group 7 duh)
gets darker in colour as you go down the group
all diatomic molecules
7 electrons in the outer shell (group 7 duh)
gets darker in colour as you go down the group
all diatomic molecules
Chl
Chorine is a toxic gas that cannot be stored easily
- reacted with potassium manganate to produce potassium chloride which is then dissolved in water which separates some of the chloride ions into chlorine
overall product=
- reacted with potassium manganate to produce potassium chloride which is then dissolved in water which separates some of the chloride ions into chlorine
overall product=
halogens with iron wool

Most reactive: fluorine
Least reactive: iodine
Reactivity decreases as you go down the group
Fluorine is the most reactive because it has the least electrions
Least reactive: iodine
Reactivity decreases as you go down the group
Fluorine is the most reactive because it has the least electrions
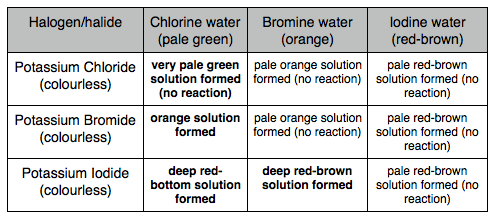
halogen: element in group 7
halide: compound of a halogen and another element
halide: compound of a halogen and another element
More reactive halogens replace halides
Group 7
Reactions
Displacement Reactions
