A level Chemistry (Inorganic Chemistry) Flashcards on Periodicity, created by Anushka John on 25/11/2016.
Pinned to
5
0
0
No tags specified
|
|
Created by Anushka John
about 8 years ago
|
|
Close
|
|
Created by Anushka John
about 8 years ago
|
|

Elements are classed as s, p or d according to what?
What happens to atomic radius as you go across (left to right) the period?
What is the general trend for 1st ionisation energy across both period 2 and 3?
What is the first anomaly in the trend of 1st ionisation energy across period 2 and 3?
What is the second anomaly in 1st ionisation energy across period 2 and 3?
Explain the trend for Na. Mg and Al
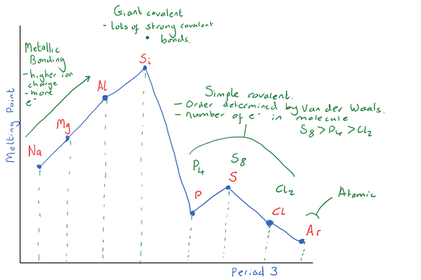
Explain why Si has the highest boiling point?
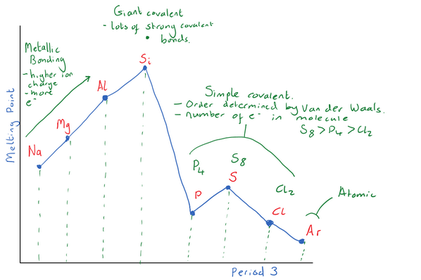
Explain the trend in boiling points for
P4(s), S8(s) and Cl2 (g)
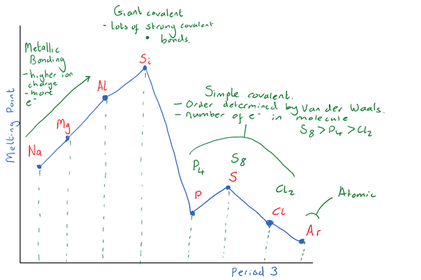
Explain why Ar has the lowest boiling point
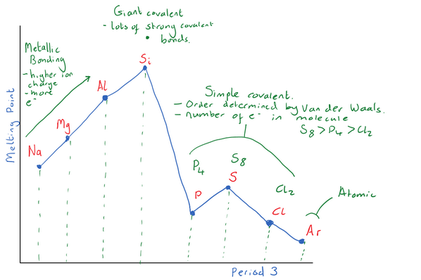
Period 2 has a similar trend in boiling points to Period 3. Describe the bonding in the different Period 2 elements and their relative boiling points.

 Hide known cards
Hide known cards